Bioinspired synthesis of pentalene-based chromophores from an oligoketone chain†
Abstract
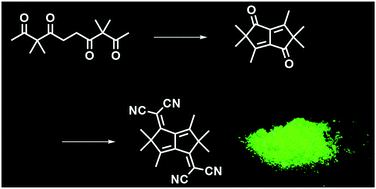
We report a bioinspired synthesis of 2,5-dihydropentalene-based chromophores from an aliphatic oligoketone bearing 1,3- and 1,4-diketone subunits. Unlike the natural polyketone sequence, fused five-membered rings were formed via an intramolecular aldol condensation. A subsequent Knoevenagel condensation reaction with malononitrile furnished a multiply cross-conjugated π-system with low-lying LUMO levels. Furthermore, pentalenes obtained from a non-conjugated aliphatic chain exhibited visible absorption and solid-state fluorescence.
- This article is part of the themed collection: 2018 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...