Easily accessible lithium compound catalyzed mild and facile hydroboration and cyanosilylation of aldehydes and ketones†
Abstract
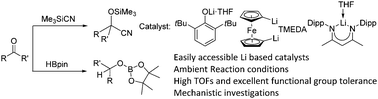
Simple and readily accessible lithium compounds such as 2,6-di-tert-butyl phenolate lithium (1a), 1,1′ dilithioferrocene (1b) and nacnac lithium (1c) are found to be efficient single site catalysts for hydroboration of a range of aldehydes and ketones with HBpin at room temperature. The efficacy of 1a–1c as catalysts is extended to the cyanosilylation of aldehydes and ketones with Me3SiCN.
- This article is part of the themed collection: 2018 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...