Chiral separation and characterization of triazatruxene-based face-rotating polyhedra: the role of non-covalent facial interactions†
Abstract
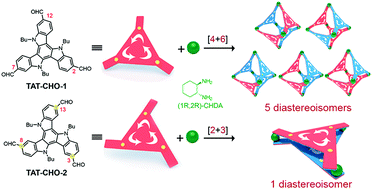
We constructed a series of novel chiral molecular face-rotating polyhedra (FRP) from two 10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole (triazatruxene) derivatives and trans-1,2-cyclohexane diamine, and investigated how facial interactions and the positions of substituents determine the diastereoselectivity and geometry of the final assemblies.


 Please wait while we load your content...
Please wait while we load your content...