N-Heterocyclic carbene-catalyzed annulation of ynals with amidines: access to 1,2,6-trisubstituted pyrimidin-4-ones†
Abstract
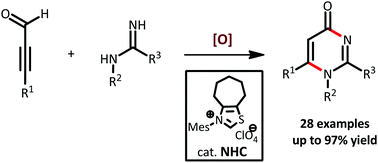
An N-heterocyclic carbene-catalyzed annulation of ynals and amidines has been reported to construct pyrimidin-4-ones. The protocol features a broad substrate scope and mild conditions. Furthermore, an oxidative strategy to catalytically generate ynal-derived acyl azolium intermediates is discussed in this mechanistic study.


 Please wait while we load your content...
Please wait while we load your content...