Electrochemical amphotericity and NIR absorption induced via the step-wise protonation of fused quinoxaline-tetrathiafulvalene-pyrroles†
Abstract
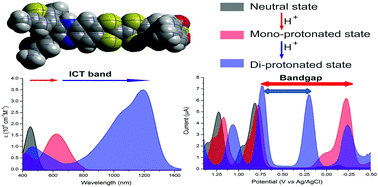
We describe an effective approach to producing electrochemical amphoteric character and tuning optical properties. Reversible step-wise protonation of quinoxaline annulated TTF-pyrrole derivatives promotes intramolecular electron-transfer and leads to formation of stable, fully charge-separated diradical states. This allows for the creation of low bandgap systems and NIR optical properties.


 Please wait while we load your content...
Please wait while we load your content...