Efficient peptide ligation between allyl-protected Asp and Cys followed by palladium-mediated deprotection†
Abstract
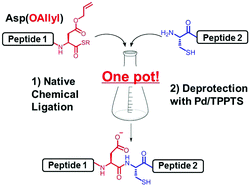
An efficient method for peptide ligation between C-terminal Asp(OAllyl) and N-terminal Cys has been developed. Peptide ligation and removal of the allyl group at the Asp carboxylate side chain proceeded in one pot by adding a small amount of Pd/TPPTS complex. Based on this efficient synthetic method, PEP-19 (61 amino acids), which is highly expressed in Purkinje cells, was synthesized.


 Please wait while we load your content...
Please wait while we load your content...