The reductive aromatization of naphthalene diimide: a versatile platform for 2,7-diazapyrenes†
Abstract
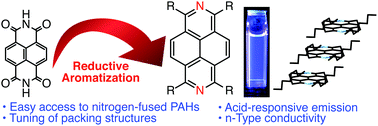
The reductive aromatization of naphthalene diimide provides tetrapivaloxy-2,7-diazapyrene, which serves as a versatile platform toward peripherally substituted 2,7-diazapyrenes. Time-resolved microwave conductivity measurements demonstrated that the intrinsic electron mobility of 2,7-diazapyrene is significantly higher than that of the corresponding pyrene.


 Please wait while we load your content...
Please wait while we load your content...