Coordination chemistry within a protein host: regulation of the secondary coordination sphere†
Abstract
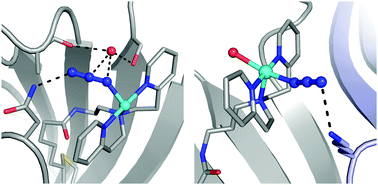
Secondary coordination spheres of metal complexes are instrumental in controlling properties that are linked to function. To study these effects in aqueous solutions artificial Cu proteins have been developed using biotin–streptavidin (Sav) technology and their binding of external azide ions investigated. Parallel binding studies were done in crystallo on single crystals of the artificial Cu proteins. Spectroscopic changes in solution are consistent with azide binding to the Cu centers. Structural studies corroborate that a Cu–N3 unit is present in each Sav subunit and reveal the composition of hydrogen bonding (H-bonding) networks that include the coordinated azido ligand. The networks involve amino acid residues and water molecules within the secondary coordination sphere. Mutation of these residues to ones that cannot form H-bonds caused a measurble change in the equilibrium binding constants that were measured in solution. These findings further demonstrate the utility of biotin–Sav technology to prepare water-stable inorganic complexes whose structures can be controlled within both primary and secondary coordination spheres.


 Please wait while we load your content...
Please wait while we load your content...