Chemoselective amide reductions by heteroleptic fluoroaryl boron Lewis acids†
Abstract
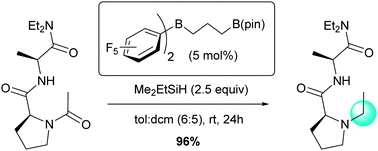
The heteroleptic borane catalyst (C6F5)2B(CH2CH2CH2)BPin is found to hydrosilylatively reduce amides under mild conditions. Simple tertiary amides can be reduced using Me2EtSiH, whereas tertiary benzamides required a more reactive secondary silane, Et2SiH2, for efficient reduction. The catalytic system described exhibits exceptional chemoselectivity in the reduction of oligoamides and tolerates functionalities which are prone to reduction under similar conditions.


 Please wait while we load your content...
Please wait while we load your content...
