A 2-(1-methylhydrazinyl)pyridine-directed C–H functionalization/spirocyclization cascade: facile access to spirosuccinimide derivatives†
Abstract
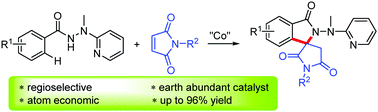
A cobalt-catalyzed oxidative coupling of benzoic hydrazides with maleimides by utilizing 2-(1-methylhydrazinyl)pyridine as a bidentate directing group has been developed. This C–H functionalization/spirocyclization cascade protocol shows high efficiency and remarkable functional group tolerance, and the ubiquitous spirosuccinimides were obtained in good to excellent yields with high regioselectivity. This strategy also provides a novel and efficient access to diverse symmetric and unsymmetrical bisspirosuccinimides.


 Please wait while we load your content...
Please wait while we load your content...