Making good on a promise: ionic liquids with genuinely high degrees of thermal stability†
Abstract
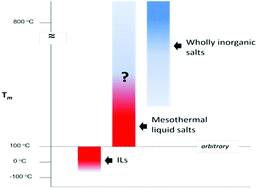
Thermally robust materials have been of interest since the middle of the past century for use as high temperature structural materials, lubricants, heat transfer fluids and other uses where thermal stability is necessary or desirable. More recently, ionic liquids have been described as ‘thermally robust,’ with this moniker often originating from their low volatility rather than their innate stability. As many ionic liquids have vanishingly low vapor pressures, the upper limit of their liquid state is commonly considered to be their degradation temperature, frequently reported from TGA measurements. The short duration ramps often used in TGA experiments can significantly overestimate the temperature at which significant degradation begins to occur when the compounds are held isothermal for even a few hours. Here, we review our recent work, and that of colleagues, in developing thermally robust ionic compounds, primarily perarylphosphonium and perarylsulfonium bistriflimide salts, in some of which cation stability exceeds that of the anion. We have used a combination of molecular design, synthesis, and computational modeling to understand the complex tradeoffs involving thermal stability, low melting point and other desirable physicochemical properties.
- This article is part of the themed collection: Ionic Liquids in the Synthesis, Fabrication, and Utilization of Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...
