Simple bond patterns predict the stability of Diels–Alder adducts of empty fullerenes†
Abstract
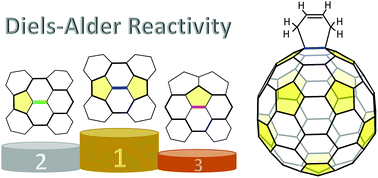
We present an extensive and systematic study on the regioselectivity of Diels–Alder (DA) cycloadditions to empty fullerenes, covering the whole range of cage sizes from C60 to C180. Reaction energies obtained from DFT calculations, which correlate with activation barriers, can be well reproduced by using a simple Hückel model, indicating that π electronic effect is the key factor determining the relative stability of DA adducts. Based on these results, we propose a couple of simple rules of thumb, in terms of a set of bond patterns, as a visual guide for approximate prediction of DA reactive sites. Moreover, we suggest two quantitative descriptors for the stability of DA regioadducts of empty fullerenes; one combines the π free valences and bond orders involved in the DA addition, and the other characterizes the local π aromaticity around the addition site. The latter criterion allows us to easily rationalize the proposed rules.


 Please wait while we load your content...
Please wait while we load your content...