High pressure NMR reveals conformational perturbations by disease-causing mutations in amyloid β-peptide†
Abstract
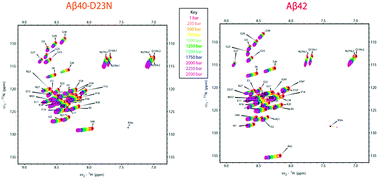
Here we present the high pressure NMR characterization of Aβ42 and two Aβ40 variants with Alzheimer-causing mutations E22G and D23N. While chemical shifts only identified localized changes at ambient pressure compared with Aβ40, high pressure NMR revealed a common site with heightened pressure sensitivity at Q15, K16 and L17 in all three variants, which correlates to higher β-propensity at central hydrophobic cluster (CHC) and faster aggregation.
- This article is part of the themed collection: Amyloid Aggregation


 Please wait while we load your content...
Please wait while we load your content...