Direct access to a cAAC-supported dihydrodiborene and its dianion†
Abstract
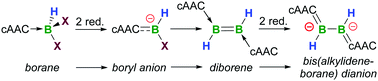
The two-fold reduction of (cAAC)BHX2 (cAAC = 1-(2,6-diisopropylphenyl)-3,3,5,5-tetramethylpyrrolidin-2-ylidene; X = Cl, Br) provides a facile, high-yielding route to the dihydrodiborene (cAAC)2B2H2. The (chloro)hydroboryl anion reduction intermediate was successfully isolated using a crown ether. Overreduction of the diborene to its dianion [(cAAC)2B2H2]2− causes a decrease in the B–B bond order whereas the B–C bond orders increase.


 Please wait while we load your content...
Please wait while we load your content...