Pt-Free microengines at extremely low peroxide levels†
Abstract
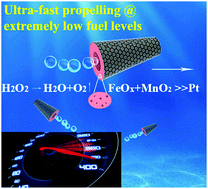
Herein, we demonstrate that iron oxide modified MnO2 (FeOx–MnO2) catalyzed micromotors can be fabricated via electrochemical co-reduction and exhibit exceptional high performance at an extremely low hydrogen peroxide (H2O2) fuel concentration. We observed that graphene/FeOx–MnO2 microtubes could show motion behaviors at fuel concentration as low as 0.03% H2O2, which is nearly one order of magnitude lower than Pt-based micromotors (normally at above 0.2% H2O2). Moreover, the micromotors exhibit higher speeds than any other reported catalytic micro/nanomotors (MNMs) at low peroxide levels. The FeOx–MnO2 systems are better catalytic MNMs, due to their excellent catalytic activity, easy fabrication, robust structure and movement, as well as low-cost, biocompatible and abundance nature, showing great potential for future applications.


 Please wait while we load your content...
Please wait while we load your content...