Synthesis of spiroindolenines by intramolecular ipso-iodocyclization of indol ynones†
Abstract
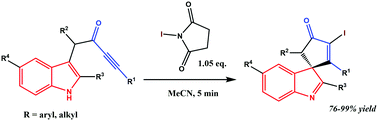
A high-yielding fast spirocyclization of easily available indol ynones has been developed by applying N-iodosuccinimide. The formation of the desired product occurs in an atom-economical way, under mild conditions, instantly after the addition of the reagent. The expected 1,2-rearrangement was not observed. The procedure represents a metal free spirocyclization of indoles with an opportunity for further functionalizations.


 Please wait while we load your content...
Please wait while we load your content...