Inhibition of amyloid Aβ aggregation by high pressures or specific d-enantiomeric peptides†
Abstract
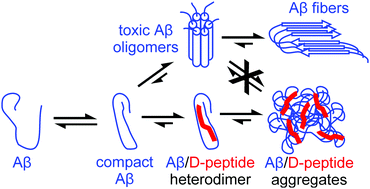
Pressure can shift the polymer–monomer equilibrium of Aβ, increasing pressure first leads to a release of Aβ-monomers, surprisingly at pressures higher than 180 MPa repolymerization is induced. By high pressure NMR spectroscopy, differences of partial molar volumes ΔV0 and compressibility factors Δβ′ of polymerization were determined at different temperatures. The D-enantiomeric peptides RD2 and RD2D3 bind to monomeric Aβ with affinities substantially higher than those determined for fibril formation. By reducing the Aβ concentration below the critical concentration for polymerization they inhibit the formation of toxic oligomers. Chemical shift perturbation allows the identification of the binding sites. The D-peptides are candidates for drugs preventing Alzheimer's disease. We show that RD2D3 has a positive effect on the cognitive behaviour of transgenic (APPSwDI) mice prone to Alzheimer's disease. The heterodimer complexes have a smaller Stokes radius than Aβ alone indicating the recognition of a more compact conformation of Aβ identified by high pressure NMR before.
- This article is part of the themed collection: Amyloid Aggregation


 Please wait while we load your content...
Please wait while we load your content...