Cp*Co(iii)-catalyzed amidation of olefinic and aryl C–H bonds: highly selective synthesis of enamides and pyrimidones†
Abstract
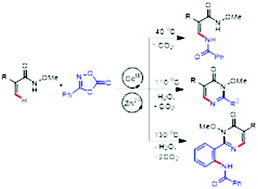
A highly efficient and selective synthesis of enamides via C–H amidation of N-methoxy acrylamides with dioxazolones is realized under [Cp*CoIII] catalysis. The resulting enamide can further selectively cyclize to form pyrimidones, which can also act as a directing group for a second C–H amidation. All these three classes of products were selectively delivered under controlled conditions.


 Please wait while we load your content...
Please wait while we load your content...