A rhodium-catalyzed transannulation of N-(per)fluoroalkyl-1,2,3-triazoles under microwave conditions – a general route to N-(per)fluoroalkyl-substituted five-membered heterocycles†
Abstract
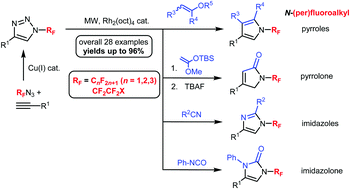
A rhodium-catalyzed transannulation via ring-opening of N-(per)fluoroalkyl-substituted 1,2,3-triazoles followed by cycloaddition with different nitriles, enol ethers, isocyanates and silyl ketene acetals under microwave heating provided a highly efficient route to previously unreported N-(per)fluoroalkyl-substituted imidazoles, pyrroles, imidazolones and pyrrolones, respectively. These reactions were found to be applicable to the synthesis of a variety of 5-membered heterocycles bearing different (per)fluoroalkyl substituents as well as both electron-donating and electron-withdrawing groups attached to the heterocyclic core.
- This article is part of the themed collection: Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...