A chiral Brønsted acid-catalyzed highly enantioselective Mannich-type reaction of α-diazo esters with in situ generated N-acyl ketimines†‡
Abstract
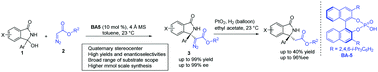
A chiral phosphoric acid-catalyzed asymmetric Mannich-type reaction of α-diazo esters with in situ generated N-acyl ketimines, derived from 3-hydroxyisoindolinones has been demonstrated in this communication. A variety of isoindolinone-based α-amino diazo esters bearing a quaternary stereogenic center were afforded in high yields (up to 99%) with excellent enantioselectivities (up to 99% ee). Furthermore, the synthetic utility of the products has been depicted by the hydrogenation of the diazo moiety of adducts.


 Please wait while we load your content...
Please wait while we load your content...