Regioselectivity inversion tuned by iron(iii) salts in palladium-catalyzed carbonylations†
Abstract
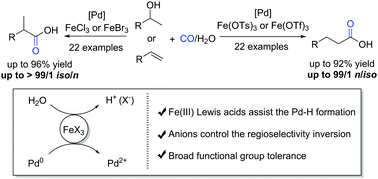
Impactful regioselectivity control is crucial for cost-effective chemical synthesis. By using cheap and abundant iron(III) salts, the hydroxycarbonylations of both aromatic and aliphatic alkenes were significantly enhanced in both reactivity and selectivity (iso/n or n/iso up to >99 : 1). Moreover, Pd-catalyzed carbonylation selectivity can be switched from branched to linear by using different Fe(III) salts. In addition, similar results were obtained for the carbonylation of secondary alcohols.


 Please wait while we load your content...
Please wait while we load your content...