Cyanotrifluoromethylthiolation of unactivated dialkyl-substituted alkynes via cyano migration: synthesis of trifluoromethylthiolated acrylonitriles†
Abstract
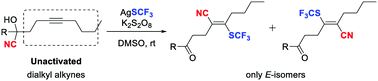
Herein a novel, elusive cyanotrifluoromethylthiolation of unactivated dialkyl-substituted alkynes is reported. Taking advantage of the intramolecular cyano migration strategy, the reaction proceeds stereoselectively to deliver E-olefinic products. A variety of tetrasubstituted trifluoromethylthiolated acrylonitriles are afforded in modest to good yields.
- This article is part of the themed collection: 2018 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...