Chemoselective deoxygenation of ether-substituted alcohols and carbonyl compounds by B(C6F5)3-catalyzed reduction with (HMe2SiCH2)2†
Abstract
B(C6F5)3-catalyzed deoxygenation of ether-substituted alcohols and carbonyl compounds has been developed using (HMe2SiCH2)2 as the reductant. This unique reagent shows distinct superiority over traditional one silicon-centered hydrosilanes, giving the corresponding alkanes in high yields with good tolerance of ethers, aryl halides and alkenes. The control experiments suggest that (HMe2SiCH2)2 might facilitate the approach in an intramolecular Si/O activation manner.
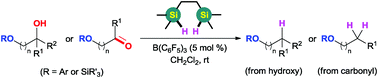


 Please wait while we load your content...
Please wait while we load your content...