A protein structure-guided covalent scaffold selectively targets the B1 and B2 subclass metallo-β-lactamases†
Abstract
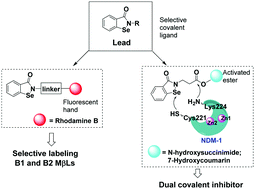
We report the discovery of ebselen-based dual covalent inhibitors of metallo-β-lactamases. Fluorescence and MALDI-TOF analysis suggested that the scaffold could bind to NDM-1 by forming a S–Se bond with Cys221 and an amide bond with Lys224 of NDM-1, thereby exhibiting selective inhibition and labeling against B1 and B2 subclass enzymes in vitro and in vivo.


 Please wait while we load your content...
Please wait while we load your content...