Palladium-catalyzed dehydrogenative coupling of cyclic enones with thiophenes: a rapid access to β-heteroarylated cyclic enones†
Abstract
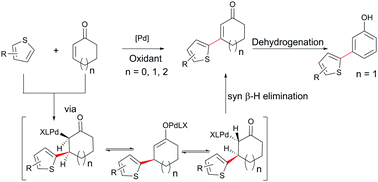
Dehydrogenative coupling of cyclic enones with heteroarenes has been a longstanding challenge because of the competitive ketone dehydrogenation and conjugated addition. Herein, a dehydrogenative coupling reaction of cyclic enones of different sizes with substituted thiophenes to construct β-thienyl cyclic enone compounds through palladium-catalyzed C–H functionalization under mild reaction conditions is reported. Simple substituted thiophenes with different functional groups can be directly introduced into cyclic enones with predominant regioselectivity at the α position of thiophene moieties and excellent functional group tolerance. Further molecular transformations of the coupling products to synthetically useful meta-heteroarylated phenol derivatives have also been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...