Brønsted base-catalyzed annulation of allyl ketones and alkynyl 1,2-diketones†
Abstract
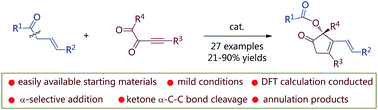
The discovery of new reaction modes mediated by easily available substrates is an important research topic in organic synthesis. Allyl ketones and related compounds have been demonstrated to undergo γ- or α-selective additions to different electrophiles. We disclose here the Brønsted base-catalyzed reaction of allyl ketones and alkynyl 1,2-diketones, which undergo a unique α-selective addition/intramolecular aldol-type annulation/C–C bond cleavage process, and a series of 2-acyloxycyclopent-3-enones can be obtained under very mild conditions.


 Please wait while we load your content...
Please wait while we load your content...