1-Bromoethene-1-sulfonyl fluoride (1-Br-ESF), a new SuFEx clickable reagent, and its application for regioselective construction of 5-sulfonylfluoro isoxazoles†
Abstract
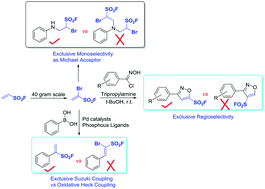
A new fluorosulfonylation reagent 1-bromoethene-1-sulfonyl fluoride was developed (1-Br-ESF). This unique reagent possesses three addressable handles (vinyl, bromide, and sulfonyl fluoride) and has great potential to function as a tris-electrophile and as a sulfur(VI) fluoride exchange (SuFEx) clickable material to enrich the SuFEx tool cabinet. The application of this reagent for regioselective synthesis of 5-sulfonylfluoro isoxazoles has been realized through a [3+2] cycloaddition with N-hydroxybenzimidoyl chlorides. This practical protocol provides a general and direct route to functionalized isoxazoles possessing sulfonyl fluoride moieties.


 Please wait while we load your content...
Please wait while we load your content...