Carbon dioxide-based facile synthesis of cyclic carbamates from amino alcohols†
Abstract
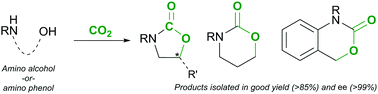
We report herein a straightforward general method for the synthesis of cyclic carbamates from amino alcohols and carbon dioxide in the presence of an external base and a hydroxyl group activating reagent. Utilizing p-toluenesulfonyl chloride (TsCl), the reaction proceeds under mild conditions, and the approach is fully applicable to the preparation of various high value-added 5- and 6-membered rings as well as bicyclic fused ring carbamates. DFT calculations and experimental results indicate a SN2-type reaction mechanism with high regio-, chemo-, and stereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...