Pd(ii)-Catalyzed asymmetric 1,6-conjugate addition of arylboronic acids to Meldrum's acid-derived dienes†
Abstract
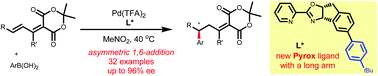
A Pd(II)-catalyzed asymmetric 1,6-addition of arylboronic acids to Meldrum's acid-derived dienes was developed. A new substituted In-Pyrox ligand was designed to enable this reaction with high enantioselectivity via a remote steric effect. A series of Meldrum's acid-derived dienes and arylboronic acids were tolerable to the reaction conditions, giving the desired adducts in moderate to high yields with good enantioselectivities (up to 96% ee). This reaction provides the first method of Pd(II)-catalyzed asymmetric 1,6-conjugate addition of arylboron.


 Please wait while we load your content...
Please wait while we load your content...