Isolation and structural characterization of a titanacyclopropane as key intermediate in the double aryl Grignard addition to 2-(arylethynyl)pyridine derivatives†
Abstract
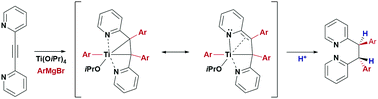
A titanacyclopropane species, which is a key reaction intermediate in the Ti(OiPr)4-mediated double aryl Grignard addition to 1,2-di(pyridin-2-yl)ethyne and related alkynes, was isolated and fully characterized. Based on this observation a one-pot synthesis of diarylated 1,2-di(pyridin-2-yl)ethanes and 2-(1,2,2-triarylvinyl)-pyridines was developed, including the tetraarylation of V-shaped 2,6-bis(arylethynyl)pyridines.


 Please wait while we load your content...
Please wait while we load your content...