Extension of antiaromatic norcorrole by cycloaddition†
Abstract
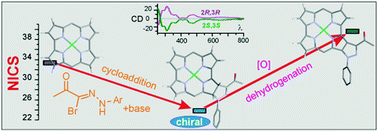
The antiaromatic ring of norcorrole, a contracted tetrapyrrolic porphyrinoid, was subjected to [2+3] dipolar cycloaddition of iminonitriles. The paratropic character of the resulting chiral chlorins was retained. The chlorins were easily dehydrogenated in the presence of air, yielding pyrazole-fused norcorroles with markedly enhanced paratropicity.


 Please wait while we load your content...
Please wait while we load your content...