Ni-catalyzed two-component reductive dicarbofunctionalization of alkenes via radical cyclization†
Abstract
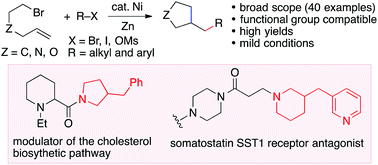
A reductive dicarbofunctionalization reaction of alkenes has been developed and applied to the preparation of substituted carbo- and heterocycles. The reaction conditions avoid the use of air-sensitive organometallic reagents, and are compatible with a broad range of bromo-electrophiles and a wide variety of substituents to give cyclic products in excellent yields.


 Please wait while we load your content...
Please wait while we load your content...