Construction of α-methoxyimidoyl ketonitrones via phosphite-mediated addition of α-keto N-tert-butanesulfinyl imidates to nitrosoarenes†
Abstract
A dimethyl phosphite-mediated addition of α-keto N-tert-butanesulfinyl imidates to nitrosoarenes was developed. Nitrosoarenes were successfully used as electrophiles to trap aza-enolate intermediates that were generated from nucleophilic addition of deprotonated phosphite to α-keto N-tert-butanesulfinyl imidates and following phospha-Brook rearrangement, allowing efficient construction of ketonitrones with excellent (Z)-geometries.
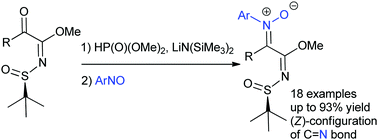


 Please wait while we load your content...
Please wait while we load your content...