Divergent synthesis of functionalized pyrrolidines and γ-amino ketones via rhodium-catalyzed switchable reactions of vinyl aziridines and silyl enol ethers†
Abstract
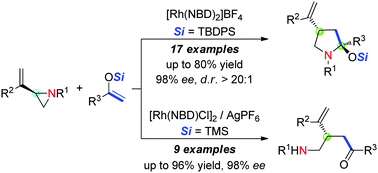
The control of reaction pathways for selective and enantiospecific synthesis of functionalized pyrrolidines and γ-amino ketones has been realized. Rhodium-catalyzed [3+2] cycloadditions of vinylaziridines and enolsilanes with a bulky silyl group gave functionalized pyrrolidines with moderate to excellent diastereoselectivities, while the reaction of silyl enol ethers with a less bulky silyl group afforded chiral γ-amino ketones in good yields.


 Please wait while we load your content...
Please wait while we load your content...