Decarbonylative thioetherification by nickel catalysis using air- and moisture-stable nickel precatalysts†
Abstract
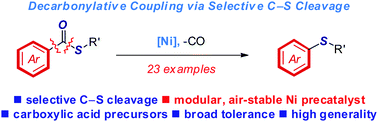
A general, highly selective method for decarbonylative thioetherification of aryl thioesters by C–S cleavage is reported. These reactions are promoted by a commercially-available, user-friendly, inexpensive, air- and moisture-stable nickel precatalyst. The process occurs with broad functional group tolerance, including free anilines, cyanides, ketones, halides and aryl esters, to efficiently generate thioethers using ubiquitous carboxylic acids as ultimate cross-coupling precursors (cf. conventional aryl halides or pseudohalides). Selectivity studies and site-selective orthogonal cross-coupling/thioetherification are described. This thioester activation/coupling has been highlighted in the expedient synthesis of biorelevant drug analogue. In light of the synthetic utility of thioethers and Ni(II) precatalysts, we anticipate that this user-friendly method will be of broad interest.


 Please wait while we load your content...
Please wait while we load your content...