Rational selection of the cation of an ionic liquid to control the reaction outcome of a substitution reaction†
Abstract
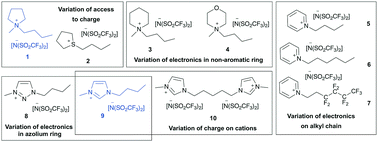
A range of ionic liquids was examined as solvents for a substitution reaction. They were chosen through rationally varying the ionic liquid cation in order to enhance the rate constant. Access to charge and electron-withdrawing substituents benefitted rate enhancement, allowing ionic liquids to be rationally selected to control reaction outcome.
- This article is part of the themed collection: Ionic Liquids in the Synthesis, Fabrication, and Utilization of Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...