Silver-mediated direct C–H amination of BODIPYs for screening endoplasmic reticulum-targeting reagents†
Abstract
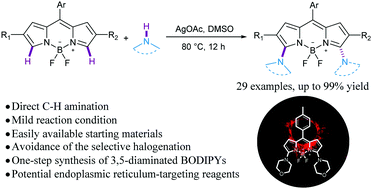
A highly efficient direct C–H amination of BODIPYs has been accomplished through the Ag(I)-mediated nucleophilic addition of an amino radical to BODIPY at the C3- and/or C5-position and deprotonation processes under mild conditions. This protocol greatly streamlines the access to a variety of 3-aminated and 3,5-diaminated BODIPYs. The resulting BODIPYs with morpholine groups (3q and 4b) exhibit specific endoplasmic reticulum (ER)-localization capacities and negligible cytotoxicities, which would be potential ER-targeting reagents.


 Please wait while we load your content...
Please wait while we load your content...