Membrane bound COMT isoform is an interfacial enzyme: general mechanism and new drug design paradigm†
Abstract
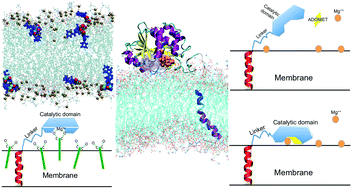
The enzyme catechol-O-methyltransferase (COMT) has water soluble (S-COMT) and membrane associated (MB-COMT), bitopic, isoforms. Of these MB-COMT is a drug target in relation to the treatment of Parkinson's disease. Using a combination of computational and experimental protocols, we have determined the substrate selection mechanism specific to MB-COMT. We show: (1) substrates with preferred affinity for MB-COMT over S-COMT orient in the membrane in a fashion conducive to catalysis from the membrane surface and (2) binding of COMT to its cofactor ADOMET induces conformational change that drives the catalytic surface of the protein to the membrane surface, where the substrates and Mg2+ ions, required for catalysis, are found. Bioinformatics analysis reveals evidence of this mechanism in other proteins, including several existing drug targets. The development of new COMT inhibitors with preferential affinity for MB-COMT over S-COMT is now possible and insight of broader relevance, into the function of bitopic enzymes, is provided.


 Please wait while we load your content...
Please wait while we load your content...