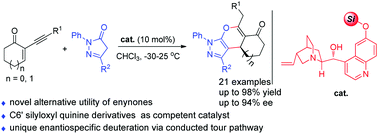
C6′ steric bulk of cinchona alkaloid enables an enantioselective Michael addition/annulation sequence toward pyranopyrazoles†
Abstract
A catalytic asymmetric Michael addition/annulation process between pyrazolones and enynones towards enantioenriched pyranopyrazoles was developed. Key features include the development of a cinchona alkaloid with C6′ steric bulk as a competent catalyst and the observation of a unique stereospecific deuteration α to the keto group via a conducted tour pathway.


 Please wait while we load your content...
Please wait while we load your content...