Stereoselective synthesis of a Podophyllum lignan core by intramolecular reductive nickel-catalysis†
Abstract
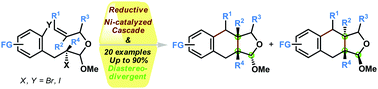
A Ni-catalyzed reductive cascade to a diastereocontrolled construction of THN[2,3-c]furan, is developed. The mild reaction conditions led to the tolerance of broad functional groups that can be placed in almost every position of this skeleton with good yields. The conformational control for the observed trans- or cis-fused selectivity during this tandem cyclization-coupling is also proposed.


 Please wait while we load your content...
Please wait while we load your content...