Copper-catalyzed decarboxylative regioselective synthesis of 1,5-disubstituted 1,2,3-triazoles†
Abstract
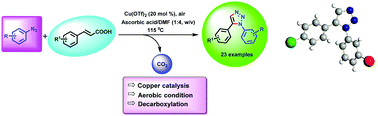
A copper-catalyzed decarboxylative regioselective protocol for the synthesis of 1,5-disubstituted 1,2,3-triazoles through direct annulation of cinnamic acids with aryl azides has been developed. This is the first example of 1,5-disubstituted 1,2,3-triazoles, under aerobic conditions using Cu(II) as the catalyst, which were generally synthesized using a ruthenium(II) catalyst. The simplicity and regioselectivity of this methodology, complementing to the classical CuAAC catalyzed the synthesis of 1,4-disubstituted 1,2,3-triazoles.


 Please wait while we load your content...
Please wait while we load your content...