Melting proteins confined in nanodroplets with 10.6 μm light provides clues about early steps of denaturation†
Abstract
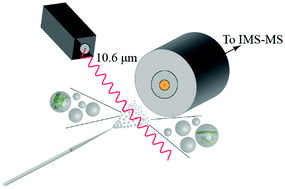
Ubiquitin confined within nanodroplets was irradiated with a variable-power CO2 laser. Mass spectrometry analysis shows evidence for a protein “melting”-like transition within droplets prior to solvent evaporation and ion formation. Ion mobility spectrometry reveals that structures associated with early steps of denaturation are trapped because of short droplet lifetimes.


 Please wait while we load your content...
Please wait while we load your content...