A combinatorial approach towards the synthesis of non-hydrolysable triazole–iduronic acid hybrid inhibitors of human α-l-iduronidase: discovery of enzyme stabilizers for the potential treatment of MPSI†
Abstract
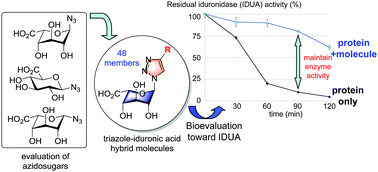
Preparation of substituent-diverse, triazole–iduronic acid hybrid molecules by click reaction of an azido iduronic acid derivative with randomly chosen alkynes is described. Library members were screened for their ability to inhibit α-L-iduronidase, and hit molecules and analogues were then investigated for their ability to stabilize rh-α-IDUA in a thermal denaturation study. This work resulted in the discovery of the first small molecules that can be used to stabilize exogenous rh-α-IDUA protein in vitro.


 Please wait while we load your content...
Please wait while we load your content...