Phase transition triggered aggregation-induced emission in a photoluminescent uranyl–organic framework†
Abstract
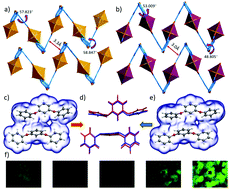
When exposed to water, the two-dimensional uranyl–organic layered compound [(CH3)2NH2][(UO2)(BCPBA)]·2DMF·H2O (H3BCPBA = 3,5-bis (4′-carboxylphenoxy) benzoic acid) gradually undergoes a complete single-crystal-to-single-crystal phase transition to [(CH3)2NH2][(UO2)(BCPBA)]·3.4H2O, resulting in an enhanced ligand–ligand interaction between the adjacent layers. This process gives rise to initial quenching of the uranyl photoluminescence followed by subsequent recovery of the photoluminescence with a much elevated intensity, as a unique case of aggregation-induced emission in an extended solid system, further confirmed by DFT analysis on bonding.


 Please wait while we load your content...
Please wait while we load your content...