Toward universal protein post-translational modification detection in high throughput format†
Abstract
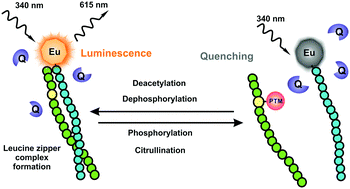
Post-translational modification (PTM) of proteins plays essential regulatory roles in a variety of pathological conditions. Reliable and practical assays are required to accelerate the discovery of inhibitors and activators for PTM related diseases. Today, methodologies are based on specific or group-specific PTM recognition of e.g. phosphate for kinase activity without extending to other type of PTMs. Here we have established a universal time-resolved luminescence assay on a peptide-break platform for the direct detection of wide variety of PTMs. The developed assay is based on the leucine zipper concept wherein a europium-chelate labeled detection peptide and a non-labeled peptide substrate form a highly luminescent dimer. As an active PTM enzyme at sub or low nanomolar concentration modifies the substrate peptide, the luminescent signal of the detached detection peptide is quenched in the presence of soluble quenchers. The functionality of this universal assay technique has been demonstrated for the monitoring of phosphorylation, dephosphorylation, deacetylation, and citrullination with high applicability also to other PTMs in a high throughput format.


 Please wait while we load your content...
Please wait while we load your content...