Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)†
Abstract
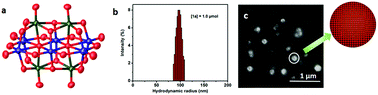
A soft-oxometalate (SOM) based on Mo and V i.e. {MoV9} in their highest oxidation state reduces CO2 to HCOOH selectively in water. Catalysis initiates without the use of any photosensitizer and solvent water acts as the sacrificial electron donor which gets oxidized to generate oxygen. Electrons and protons released in this process reduce CO2 to HCOOH.


 Please wait while we load your content...
Please wait while we load your content...