Diels–Alder reactions between hexafluoro-2-butyne and bis-furyl dienes: kinetic versus thermodynamic control†
Abstract
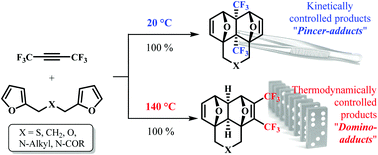
The tandem [4+2] cycloaddition between hexafluoro-2-butyne and bis-furyl dienes, like difurfuryl ester, at room temperature leads to the kinetically controlled “pincer”-adducts – annulated 4a,8a-bis(trifluoromethyl)hexahydro-1,4:5,8-diepoxynaphthalenes. On the other hand, if these reactions proceed at 140 °C, only the thermodynamically controlled “domino”-adducts – annulated 2,3-bis(trifluoromethyl)hexahydro-1,4:5,8-diepoxynaphthalenes – are formed. Therefore, a very rare and unexpected example of full kinetic and thermodynamic control in the Diels–Alder reaction is reported in this paper.


 Please wait while we load your content...
Please wait while we load your content...