Interplay of Lewis acidity, intramolecular O→Sn interactions and selectivity: organotin-functionalized crown ethers as ditopic hosts for sodium and potassium halides†
Abstract
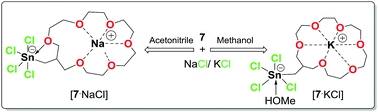
The increased Lewis acidity in organotin-functionalized crown ethers X3SnCH2[19]-crown-6 (5, X = I; 6, X = Br; 7, X = Cl) not only resulted in ditopic complexation of sodium/potassium halides, but also offers an excellent strategy to manipulate through intramolecular O→Sn interactions the selectivity of the crown ether moiety towards Na+/K+ depending on the solvent.


 Please wait while we load your content...
Please wait while we load your content...