Synthesis of indolizine derivatives containing eight-membered rings via a gold-catalyzed two-fold hydroarylation of diynes†
Abstract
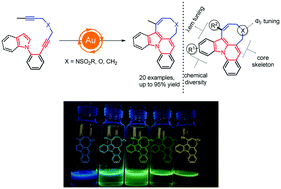
A gold-catalyzed method for the construction of indolizines with eight-membered rings has been developed. The reaction proceeded through a two-fold hydroarylation with indole or pyrrole derivatives containing a 1,6-diyne using a tri(1-adamantyl)phosphine gold complex as the catalyst, affording 1,8-disubstituted indolizines in moderate to good yields in DCE at 80 °C. The potential usefulness of these indolizines as blue or green OLEDs has been also disclosed.


 Please wait while we load your content...
Please wait while we load your content...