Alkene functionalization for the stereospecific synthesis of substituted aziridines by visible-light photoredox catalysis†
Abstract
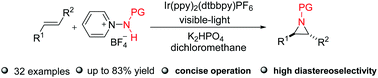
A novel strategy involving visible-light-induced functionalization of alkenes for the synthesis of substituted aziridines was developed. The readily prepared N-protected 1-aminopyridinium salts were used for the generation of N-centered radicals. This approach allowed the synthesis of aziridines bearing various functional groups with excellent diastereoselectivity under mild conditions. Moreover, this protocol was successfully applied to prepare structurally diverse nitrogen-containing frameworks.


 Please wait while we load your content...
Please wait while we load your content...